64 KiB
Influence of manufacturing process on Cavitation Erosion on CoCrWMoCFeNiSiMn (Stellite 1) alloys
Agenda
- Introduction
- Aims
- Methodology
- Results & Discussion
- Conclusion
Introduction
Cavitation Erosion
A block BMCOL
- What is cavitation? Collapse of bubbles and the resulting high-frequency high-pressure shock waves. Caused by fluid pressure dropping to vapor pressure, which is particularly common with high fluid flow speeds \cite{krellaDegradationProtectionMaterials2023}.
- Why does it matter? Cavitation erosion leads to removal of material, crack growth, and part failure. Affects turbine blades, pump impellers, valves, stirrers, etc.
A screenshot BMCOL
Influences on Stellite Properties
Influences on Stellite Properties
COMMENT Stellite
This slide consists of some text with a number of bullet points:
- the first, very @important@, point!
- the previous point shows the use of the special markup which translates to the Beamer specific alert command for highlighting text.
The above list could be numbered or any other type of list and may include sub-lists.
COMMENT Stellite mind map
This slide consists of some text with a number of bullet points:
COMMENT Why
COMMENT A simple slide
This slide consists of some text with a number of bullet points:
- the first, very @important@, point!
- the previous point shows the use of the special markup which translates to the Beamer specific alert command for highlighting text.
The above list could be numbered or any other type of list and may include sub-lists.
COMMENT A more complex slide
This slide illustrates the use of Beamer blocks. The following text, with its own headline, is displayed in a block:
Org mode increases productivity B_theorem
- org mode means not having to remember LaTeX commands.
- it is based on ascii text which is inherently portable.
- Emacs!
\hfill \(\qed\)
COMMENT Two columns
A block BMCOL
- this slide consists of two columns
- the first (left) column has no heading and consists of text
- the second (right) column has an image and is enclosed in an example block
A screenshot BMCOL B_example
Stuff
Aims
Methodology
Methodology - ASTM G32 Cavitation Erosion Testing
Text BMCOL
Naturally aerated seawater at room temperature.
Figure of ASTMG32 standard BMCOL
Methodology - ASTM G32 Cavitation Erosion Testing
Close up picture of cavitation erosion BMCOL
Figure of analytical balance BMCOL
Figure of sample holder BMCOL
Methodology - ASTM G32 Cavitation Erosion Testing
Text BMCOL
- Seawater was vacuum filtered in order to remove algae and suspended particles
- Seawater pH was measured after calibrating pH meter with buffer solutions of pH 7 and pH 14.
Figure of Electrochemical setup BMCOL
Methodology - Electrochemical Setup
Text BMCOL
- Instrument: Corrtest CS310 Potentiostat connected to conventional three-electrode cell.
- Working Electrode (WE): The sample, with an exposed area of $2{cm}^{2}$.
- Reference Electrode (RE): Saturated Calomel Electrode (SCE).
- Counter Electrode (CE): Graphite plate.
- Electrolyte: Naturally aerated seawater at room temperature.
Figure of Electrochemical setup BMCOL
Methodology - Electrochemical Setup
Figure of Electrochemical setup BMCOL
Figure of Electrochemical setup BMCOL
Figure of Electrochemical setup BMCOL
Methodology - Electrochemical Tests
- Open Circuit Potential (OCP) Before each electrochemical test, OCP was measured for one hour to ensure each sample reaches equilibrium, before EIS and LPR (explained below).
-
Electrical Impedence Spectroscopy (EIS)
The electrical response of the sample's interface with naturally aerated seawater
- Frequency - 105 Hz → 10-1 Hz
- Excitation voltage - 10 mV and 20 mV
- Spacing - 20 per decade, logarithmic
-
Linear Polarization Curve (LPR)
The current density through the sample with an externally imposed voltage
- Voltage - -20 mV wrt OCP → 20 mV wrt OCP
- Scan rate - 0.1 mV/s
- Data Acquisition rate - 10 Hz
Methodology - X-ray Diffraction (XRD)
Text BMCOL
The constituent phases were examined by X-ray diffraction
- Cu $K\alpha$ radiation ($\lambda = \qty{1.5406}{\angstrom}$),
- Bragg-Brentano $\theta{}:2\theta{}$,
- diffraction angle range $2\theta \in \left[10^\circ,80^\circ\right]$,
- step size of $0.02^\circ$,
- scanning time of 0.5 sec/step,
- sample rotation enabled
Figure of XRD instrument BMCOL
Methodology - Optical Microscopy (OM) & Electron Microscopy (SEM)
Text BMCOL
-
Optical Microscopy (OM)
Images were taken with Amscope metallurgical optical microscope
- eyepiece magnification 10X
- auxiliary magnification 5X, 10X, 20X, 50X, 100X
-
Scanning Electron Microscopy (SEM) Images were taken with Vega TESCAN and Oxford Instruments
- Secondary Emission (SE)
- Backscattered Electrons (BSE)
- Energy Dispersive X-ray Spectroscopy (EDS)
Figure of SEM instrument BMCOL
COMMENT Methodology - Scanning Electron Microscope (SEM)
Text BMCOL
Ma The constituent phases were examined by X-ray diffraction
- Cu $K\alpha$ radiation ($\lambda = \qty{1.5406}{\angstrom}$),
- Bragg-Brentano $\theta{}:2\theta{}$,
- diffraction angle range $2\theta \in \left[10^\circ,80^\circ\right]$,
- step size of $0.02^\circ$,
- scanning time of 0.5 sec/step,
- sample rotation enabled
Figure of XRD instrument BMCOL
Results & Discussion
Results - OM Images
Results - HIPed Stellite 1 SEM Images
Results - HIPed Stellite 1 SEM Images
Results - OCP
Results - OCP
A block BMCOL
- OCPs shows upward trend Upward (nobler) trend indicates passivation (formation of protective layer), reducing rate of corrosion.
- HIPed S1 has lower OCP than as-cast S1 Lower OCP is indicative of more thermodynamically stable materials \cite{ogunlakinMicrostructuralElectrochemicalCorrosion2025, rosalbinoCorrosionBehaviourAssessment2013}.
A screenshot BMCOL B_example
Results - EIS
Results - EIS
Results - EIS
Results - EIS
A block BMCOL
- HIPed S1 shows lower resistance than HIPed S6
- as-Cast S1 shows lower resistance than as-Cast S6
- HIPing, Laserclad (high cooling rate) results in higher resistance than as-Cast, PTA (low cooling rate)
- HIPing significantly improves corrosion resistance
A screenshot BMCOL B_example
Results - Tafel
Results - Tafel
A block BMCOL
- HIPed S1 shows lower resistance than HIPed S6
- as-Cast S1 shows lower resistance than as-Cast S6
- HIPing, Laserclad (high cooling rate) results in higher resistance than as-Cast, PTA (low cooling rate)
- HIPing significantly improves corrosion resistance
A screenshot BMCOL B_example
Conclusion
Effect of Casting
Effect of HIPing
Bibliography
COMMENT Preamble
Key dates
- 11 Aug – Submit abstract for presentation by 0900 UK time.
- 13 Aug – Submit presentation slides by 2359 UK time.
- 14 Aug – Presentations (timing to be confirmed).
Presentation Structure
- Title Slide - Project name, your name, supervisor name
- Agenda Slide indicating structure of talk @ high level
- Rational for work slide(s)
- Aims slide
- Methodology Slide
- Key results Slide
- Conclusion Slide
- Presentation accounts for 10% of the total grade
-
Presentation will be in-person, on Dubai campus, open to all.
- 15 minutes presentation maximum, with 10 additional minutes for Q&A and feedback.
- Print out slides as well as drafts of thesis and work report.
- Presentation must be same as submitted on Canvas.
- Recommended slide timing: 1-2 minutes per slide 12-15 slides maximum for the 15 minutes.
- Practise to ensure you do not exceed 15 minutes
- Presentation grade is the average of three academic staff assessors.
Suggestions
- Please put presentation slide number on each slide.
- One substantive point per slide; you do not need to communicate all your results.
- Not too wordy - use graphics, videos
- Not too busy - white space is good!
- Don't overdo the animation
Rationale
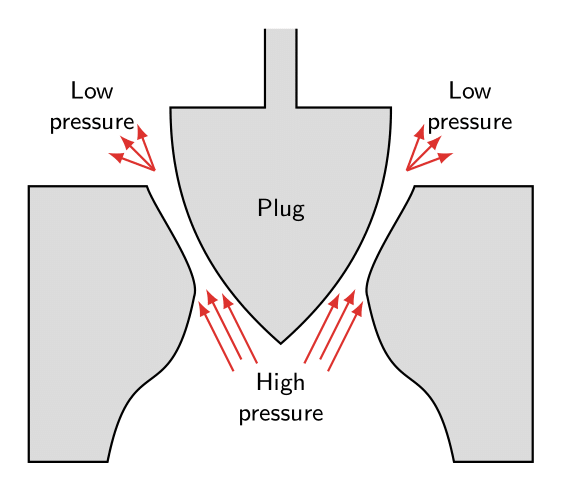
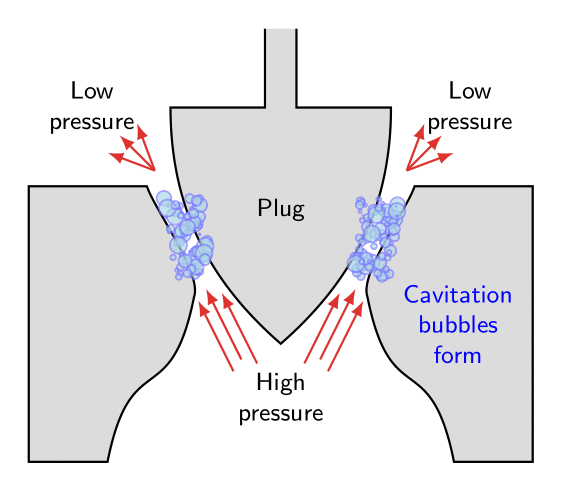
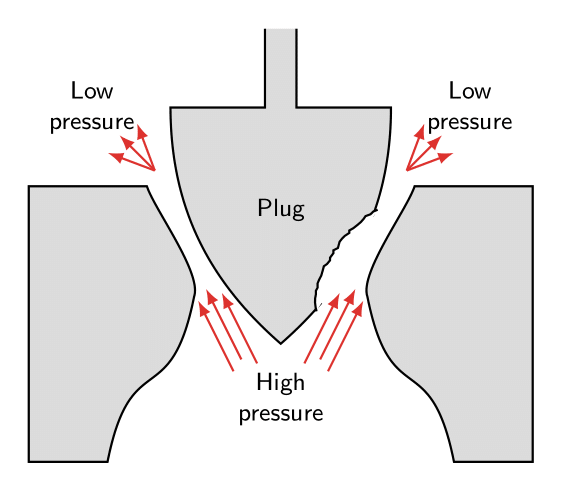
Cavitation is the formation of bubbles from small gas nuclei as the local pressure allows the flow to momentarily enter the vapor phase. When these bubbles collapse near a surface, the resulting impinging jet causes high pressures.
These bubbles subsequently collapse as they enter an area of higher pressure, as shown in the figure. The collapse acts like an implosion in which the surface is attacked by high pressure intensity of the impinging jet.
COMMENT Items to Bring to the Defense
Essentials that you should bring include:
- Your presentation
- A laser pointer
- A copy of your thesis document
- A pen or pencil
- Something to record comments
- A bottle of water